

Pi bonds effectively define your second or third covalent bond. Sigma bonds effectively define your single bond and is always present in a covalent bond.

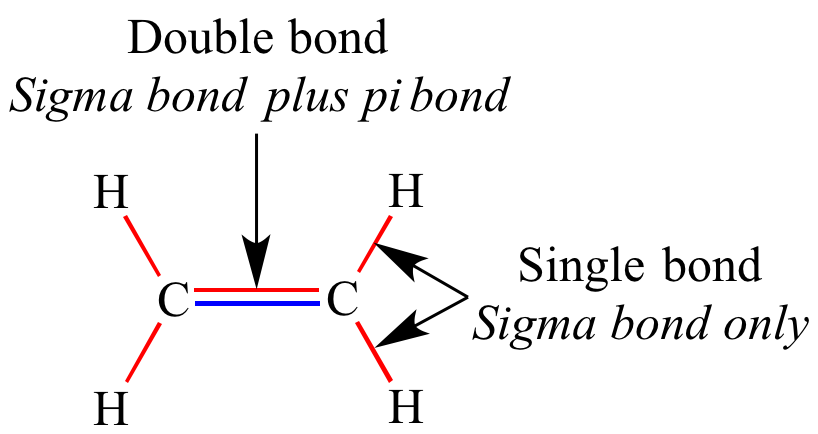

Basically, the electrons are shared in an area above and below the linear plane defined by the two atoms and their sigma bond. Pi bonds are the result of overlapping electrons in the P orbitals of the two respective atoms. Basically, the electrons are shared in an area between the two atoms. Sigma bonds are the result of overlapping electrons in the S orbital of two respective atoms. This is laymen's terms, but hopefully should be applicable for everything you'll need in your chem. Sigma bonds are normally made by s orbitals or p,z orbitals and pi bonds by p,x and p,y orbitals, however, some molecules get d or f orbitals involved too. To recap, if the actual area of overlap between the atoms looks like a stretched s orbital it is a sigma bond and if it looks like a stretched p orbital it is a pi bond. Similarly if the bond is formed from p,x or p,y orbitals the bond will be shaped like two rugby balls one above the z-axis and one below (formed from the two lobes of the p orbitals), now if you rotate the the bond about the z-axis it will transform in a similar way to a p orbital (imagine looking down the z-axis and spinning it clockwise, all you would see is something that looked like a p orbital, hence they are called pi bonds). These are all called sigma bonds because with respect to the z-axis they rotate in the same way as s orbitals. Now if the bond is created by the overlap of two s orbitals the bond will be shaped like a sort of rugby ball (or american football i'm from the UK) and if we spin the bond about the z-axis there will be no change in its appearance (it's symmetrical with respect to the z-axis), the same situation occurs if the bond is formed by p,z orbitals or any combination of s and p,z orbitals (or even d,z 2 orbitals). Now, bonding, if you imagine two nuclei sitting in space with a bond formed between them, an imaginary line connecting the two nuclei can be called the z-axis (or 'bond line' call it whatever you want). The p orbital obviously isn't spherical in shape like the s orbital, it has two great big lobes sticking out (of opposite phase, but don't worry too much about this). what on earth am I actually going on about?Īn s orbital is spherically symmetrical by which we mean if you were sat at the centre of the orbital you could look outwards in any direction and what you see is exactly the same, there would be no difference in what you saw no matter which direction you were looking, an s orbital looks like a ball or sphere.Ī p orbital on the other hand has a two dimensional plane that runs through the middle of it and divides the orbital into two tear drop shapes with absolutely zero electron density/chance of seeing an electron on the plane where the teardrops meet. There are also delta bonds which are analogous in symmetry to d orbitals. Sigma and pi are actually names derived from the symmetry of the bonds in the same way as s and p orbitals (sigma bonds and s orbitals, and, pi bonds and p orbitals).


 0 kommentar(er)
0 kommentar(er)
